Flavours and Aroma’s
The flavour and aroma of cognac are caused by a large number of chemical substances. These substances may originate from the grape itself or be created during the production process. New flavour and aroma substances can be created both during fermentation, during distillation and during maturation. The wood plays an important role during maturation, as the alcohol interacts with the wood to extract certain substances. Toasting the wood also plays an important role.
For a list of all the chemicals you can find in cognac: table of chemicals .
THE GRAPE
A grape consists mostly of its sugars, mainly glucose and fructose. During fermentation, these are converted into alcohol and carbon dioxide.
The second most important component is the organic acids. They are responsible for the sour taste. They are mainly tartaric acid, malic acid and citric acid. 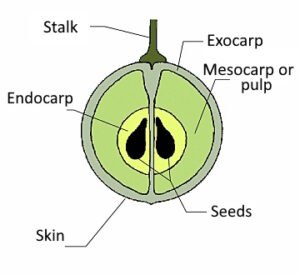 Both sugars and acids are mainly located in the pulp of the grape: the mesocarp and endocarp. (The mesocarp and endocarp of a grape are difficult to distinguish).
Both sugars and acids are mainly located in the pulp of the grape: the mesocarp and endocarp. (The mesocarp and endocarp of a grape are difficult to distinguish).
Phenols come third. They are important for colour and (tart) flavour and are mainly found in the pips and skin (exocarpium). They are mainly anthocyanins (flavonoids, colouring agents) and tannins (but also lignins).
Next come nitrogen compounds: ammonium cations and organic nitrogen compounds such as amino acids, peptides and proteins. They provide nutrition for yeast cells and lactic acid bacteria during the fermentation process.
Then there are some flavourings that are mainly located in or just under the skin. Incidentally, these are by no means always flavour compounds that are desired.
The grape also contains minerals that were absorbed from the soil. Potassium is the most important, but also sodium, iron, phosphate, sulphate and chloride.
Finally, there are the pectins found in the cell wall. These are complex polysaccharides.
THE FERMENTATION PROCESS
Previously, fermentation took place completely naturally, i.e. as a result of yeast cells living on the grape skin, usually the saccharomyces cerevisiae. Nowadays, added yeast cells, including a strain of saccharomyces cerevisiae, are almost always used for fermentation. The main function of the fermentation process is, of course, the conversion of sugars into alcohol, which also releases carbon dioxide and heat. Only glucose and fructose are converted into alcohol; other sugars (maltose, xylose, arabinose, galactose, melibiosis, raffinose and stachyose) are not converted.
Only glucose and fructose are converted into alcohol; other sugars (maltose, xylose, arabinose, galactose, melibiosis, raffinose and stachyose) are not converted.
The picture on the right shows bubbling must. The bubbles are caused by the release of carbon dioxide.
In addition, most of the volatile components present in alcohol are also formed during fermentation. The type of yeast cells used for this purpose has a big influence on exactly which volatile components are formed. They are alcohols, acid esters, aldehydes, ketones, acetals, phenols, hydrocarbons, sulphur compounds, lactones, sugars and a few others.
If the must is still too acidic after alcoholic fermentation, another malolactic fermentation can take place where the sharper malic acids are converted into the softer lactic acid. This is not actually fermentation, as it is bacteria that do the work here.
DISTILLING
Several chemical processes take place during distillation: hydrolysis of esters and of glycosides, esterification with formation of aldehydes, Maillard reactions (reaction between amino acids and reducing sugars), formation of furfural by decomposition of pentoses, formation of glycerol by the action of the copper of the kettle on fatty substances and formation of amino acids (Strecker amino acid synthesis).
The difference in distilling with lees or without lees has quite an impact on the amount of esters formed. Generally, musts are distilled without the lees, but some large houses do distill ‘sur lie’.
Low boiling point and high boiling point components are separated and kept separate. They can be reused in a subsequent distillation or sometimes discarded. At the beginning of distillation, one mainly catches fatty acid esters, aldehydes and acetals as well as the higher alcohols. They give heaviness to the eau de vie and are set aside.
In the second distillation, mainly fatty acid esters and higher alcohols are captured at the beginning, which are again set aside.
When distilling, a number of things are important that greatly influence flavour: the shape of the chapiteau (helmet) and the col de cygne (swan’s neck) determine the speed at which volatiles rise and are carried away to condense. The cut-off point (coupe) between les têtes, le coeur and les secondes determines how many highly volatile substances and highly dense substances eventually enter the eau de vie. Determining the cut-off point is still done with the nose; by smelling, the experienced Maître de Chai knows when there are undesirable substances in the distillate.

THE MATURATION PROCESS
Toasting of the cask
The eau-de-vie matures in oak barrels. These barrels are already ‘toasted’ in the tonnelier. This involves roasting the barrels on the inside by a fire. This process is called bousinage. The degree of roasting largely determines the ratio of different aromas given off by the wood. Usually, these chauffe’s, as they are called, are divided into légère or douce (light), moyenne (medium) and forte (intense). Sometimes there are more options and moyenne is further subdivided into moyenne plus, moyenne longue and medium forte. If the lids are also toasted: tres forte. The lids make up 1/3rd of the wood surface in contact with the eau-de-vie.

The purpose of toasting is mainly to release wood aromas such as vanilla, coconut and toasted bread and to reduce bitterness. What actually happens is that the macromolecules, polymers such as cellulose, hemicellulose and lignin, are broken down into smaller aromatic molecules that can be more easily absorbed into the eau-de-vie.
In general, wood is composed as follows: 42% cellulose (a polysaccharide or polymer of glucose), 26% lignin (consisting of cross-linked phenolic polymers), 27% hemicellulose (a polymer of some sugars other than glucose) and 11% extractables such as tannins, aromatics, lipids, lactones and hydrocarbons.
Roasting thus releases important aromas.
Important non-volatile components are:
- Phenolic acids, oa gallic acid (bitter and astringent) and ellagic acid (bitter);
- coumarin derivatives such as scopoletin and esculetin (mild acid)
- ellagitannins (astringent)
- dyes (quinones and semi-quinones)
Volatile toast aromas are:
- from polysaccharides, the volatile furan aldehydes (furfurols: almond, toasted bread, toasted almond, cream) and volatile furan derivatives (grassy and hay-like)
- other polysaccarides form caramel aromas (cyclotene, maltol, iso-maltol)
- lignin and polyols form volatile phenols (smoky, spicy, clove, fire tones, tar, ink), including guiacol and derivatives, syringol and derivatives, eugenol, cresol, phenol and volatile phenolic aldehydes such as vanillin, coniferous aldehyde, sinapaldehyde and syringaldehyde (weakly sweet wood odour)
- lipids, fatty acids and hydroxycarboxylic acids form isomers of methyl-octalactone (or the whisky lactone) after toasting.
The main wood scents are:
- Whiskylactone (coconut, oak), a lactone or cyclic ester
- Eugenol (clove), a volatile phenol
- Vanillin (vanilla), a phenolic aldehyde
The effects of toasting
Light toasting:
- Brings out fruit aromas. Wood aromas soften
- vegetal aromas (green and vegetable) are reduced
- light vanilla flavour and light toasted aromas
- tannin structure is improved.
Medium toast:
- fruit is dominant, complex aromas
- Vegetal aromas are pushed back even further and more complex notes emerge, including coconut aroma and vanilla and toasty notes, coffee and freshly toasted bread
- Lovely sweet oak flavours, spices (cloves), butterscotch, vanilla, caramel and chocolate.
Medium plus toast:
- Maximum yield of vanilla notes, clove and hay-like notes.
Strong toasting:
- gives grilled and spicy aromas.
- Even more smoky aromas and tarry flavours, pepper, espresso, caramel
Influence of wood during maturation process.
The first thing that takes place is the extraction of some constituents from the wood, such as lignin, tannins and haemicellulose under the action of the alcohol on the wood. The extent to which the wood was toasted when the barrel was prepared now has a great influence on the composition of the constituents that are extracted, but also, for example, how long the wood was allowed to dry before being used has an influence.
Of course, the ratio of wood surface area to volume of the eau-de-vie is also important.
Slowly, the colourless spirit takes on the colour of the wood and develops aromas: oak aroma and vanilla aromas.
After extraction, the eau-de-vie starts to digest the wood components, i.e. the wood components and the components of the eau-de-vie merge. The wood tannins form bonds with the grape tannins (polymerisation), softening the latter.
Over the following years, the smell of oak will give way to floral and vanilla aromas. The flavour slowly becomes softer and softer.
Still later, the typical rancio appears: mushroom nuances, damp undergrowth and walnut oil.


Comments
Flavours — No Comments
HTML tags allowed in your comment: <a href="" title=""> <abbr title=""> <acronym title=""> <b> <blockquote cite=""> <cite> <code> <del datetime=""> <em> <i> <q cite=""> <s> <strike> <strong>